(i) Why do we classify elements?
(ii) What are the two criteria used in the development of Modern Periodic Table?
Answer
(i)
We classify the elements so as to ensure a systematic and simplified study . By classification, we can study the properties of elements and their compounds properly.
(ii)
In the modern periodic table, elements were classified based on their
- atomic number
- chemical properties
(iii)
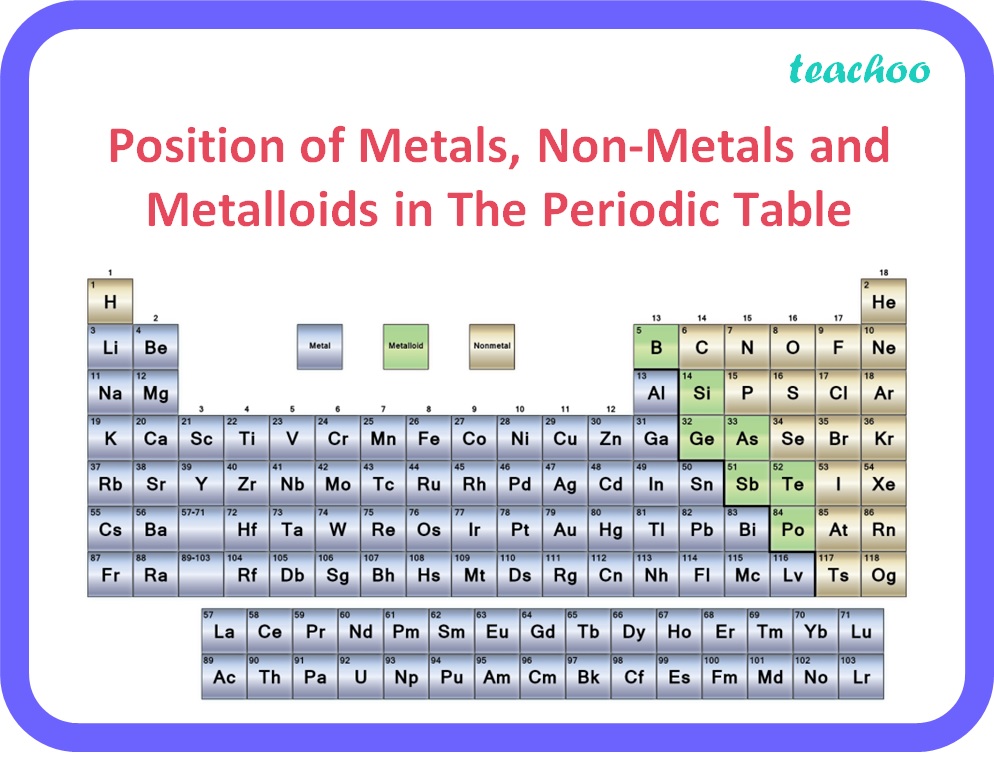
Metals are placed towards the left of the periodic table, non metals towards the right and metalloids are present in the middle in a zigzag fashion.
(iv)
In the modern periodic table, elements are classified based on their atomic number and not atomic masses .
Although isotopes have different atomic masses, they have the same atomic number and similar chemical properties.
So, they should be placed in the same slot .
