The table shows the electronic structures of four elements
| Element | Electronic Structure |
| P | 2,6 |
| Q | 2,8,1 |
| R | 2,8,7 |
| S | 2,8,8 |
a. Identify which element(s) will form covalent bonds with carbon.
Answer
Covalent Bonds are bonds formed by sharing electrons between 2 atoms .
As per the electronic configurations of the given elements, we can Identify them as:
- P- Oxygen (O)
- Q- Sodium (Na)
- R- Chlorine (Cl)
- S- Argon (Ar)
Now,
- We know that Sodium (Q) is an Alkali Metal that donates one electron to form ionic compounds. Hence, it does not form covalent bonds with Carbon.
- Argon (S) is a noble gas . Noble gases are inert elements that form neither ionic nor covalent bonds.
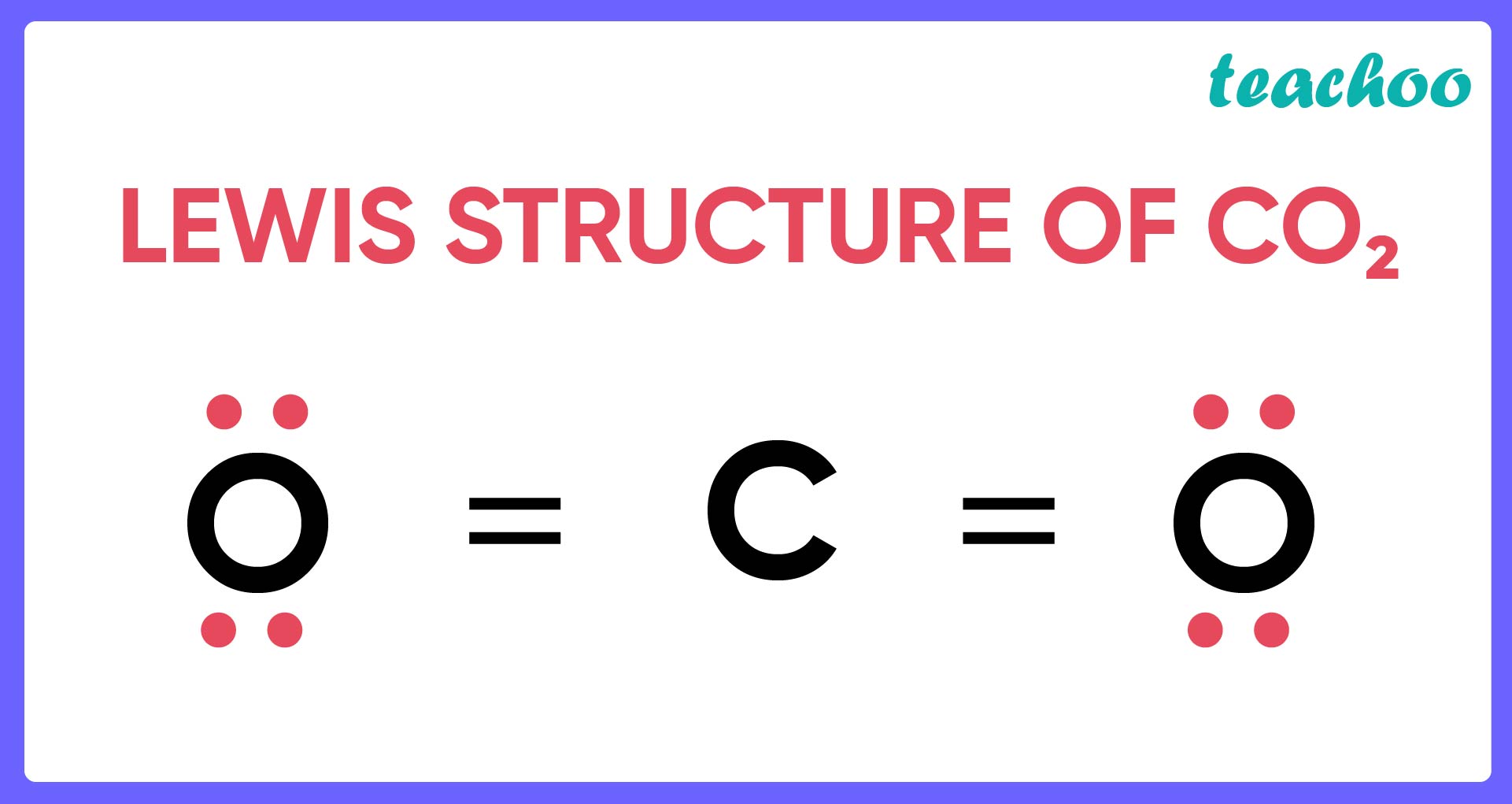
- Oxygen (P) and Chlorine (R) accept electrons to form ionic as well as covalent bonds . Oxygen bonds with Carbon to form CO 2 , while Chlorine forms CCl 4 .
So, the correct answer is P and R

b. “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
Answer
The element which is referred to here is 'P'
which is actually Oxygen (O)
Carbon reacts with Oxygen to form different compounds like
- Carbon Monoxide
- Carbon dioxide
- Carbon suboxide
Different Compounds are possible due to 2 properties of Carbon
- Tetravalancy - It means Carbon has valancy of 4 (It is tetravalent)
- Catenation - It is the property of Carbon to self combine and form long chains