What is a Solution?
It is a homogeneous mixture of 2 or more substances
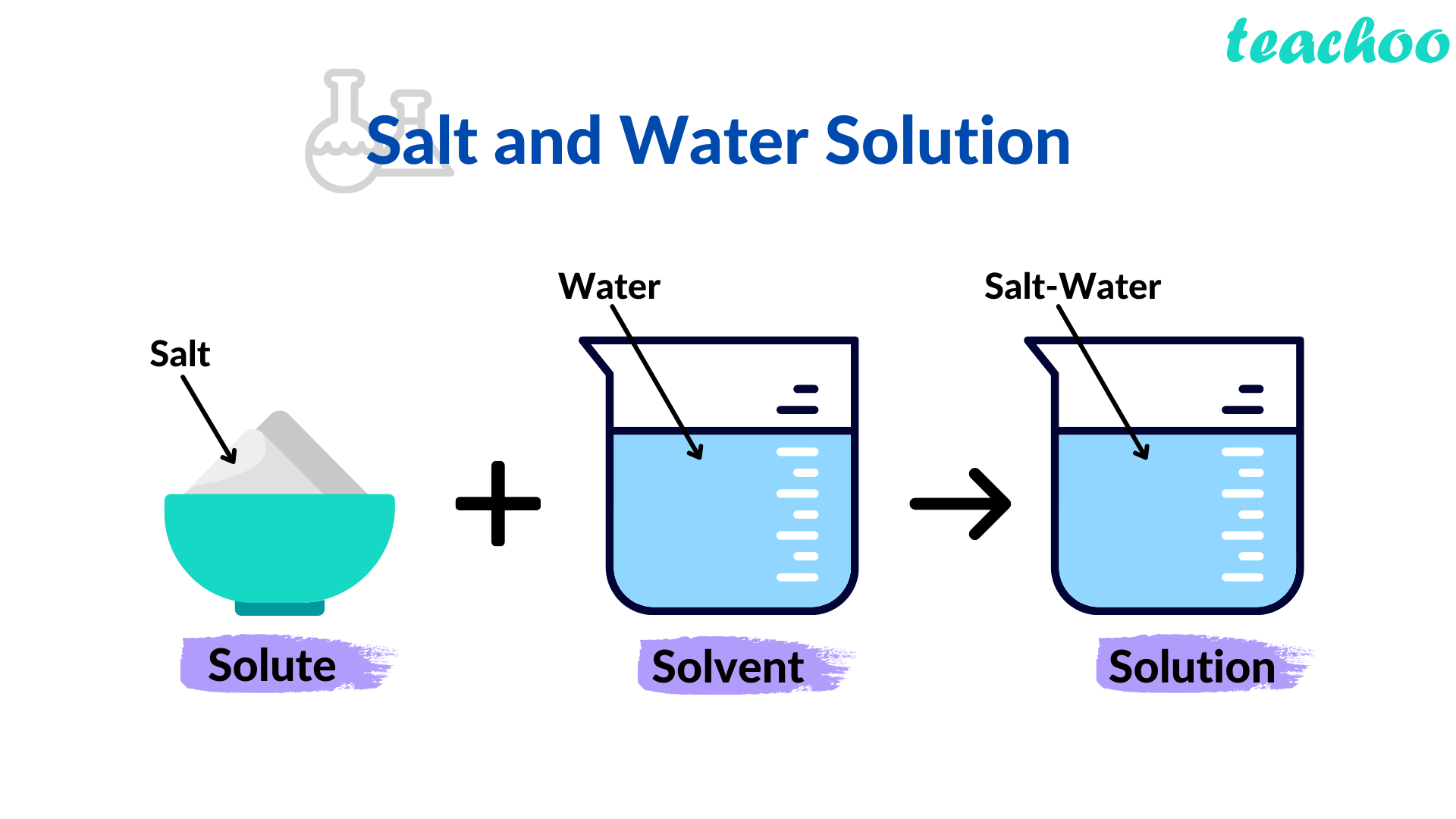
Example
- If we dissolve salt in water , it forms a solution
- Here, Salt and Water are called components of the solution
- Water is called Solvent and Salt is called Solute of a solution
Properties of a Solution
-
It is
Homogeneous Mixture
-
It is a mixture in which different substances are completely and uniformly mixed together , it is difficult to distinguish different substances from each other.
-
Particles of the solution
are very small -
They are less than 1 nm (10 -9 meter) in diameter. These are so small that they can't be seen by naked eyes
-
Particles of Solution
do not scatter a beam of light
-
If light passes through a solution, the particles of the solution don't scatter it; they do not show Tyndall Effect.
-
Solute Particles
cannot be separated by filtration -
Solute particles do not settle down. Hence, we can't use filter to separate them
Is Solution only of One Liquid and One Solid?
No,
Solution can contain any state of matter
- Gas in Liquid Solution - Aerated Drinks (Cold drinks) contain carbon dioxide (gas) as solute and flavoured water (liquid) as solvent
- Liquid in Liquid Solution - Ethyl Alcohol in Water
- Gas in Gas Solution - Air is also a solution . Main components are Nitrogen and Oxygen ( Other components are present in lesser quantities)
-
Solid in Liquid Solution
- Solution of Salt in Water (Water is solvent, Salt is solute)
- Solution of Sugar in Water (Water is solvent, Sugar is solute)
-
Solution of
Iodine in Alcohol
(Alcohol is solvent,Iodine is Solute) - Also called
tincture of Iodine
