Unbalanced Chemical Equation
An unbalanced chemical equation has an unequal number of atoms of different elements in reactants and products.
No. of atoms of elements in Reactants ≠ No. of atoms of elements in Products
An unbalanced chemical equation is also called a skeletal chemical equation
.
Example
Hydrogen (H 2 ) Reacts with Oxygen (O 2 ) to Form Water (H 2 O)
H 2 + O 2 → H 2 O

In this case,
No. of Atoms of Hydrogen in Reactant = 2
No. of Atoms of Hydrogen in Product = 2
In case of of Hydrogen , the number of atoms in reactants and products are Equal
But
No of Atoms of Oxygen in Reactant = 2
No of Atoms of Oxygen in Product = 1
In case of Oxygen , both are not equal
Since atom of all elements are not equal in Reactants and Products
It is an Unbalanced Chemical Equation
An unbalanced chemical equation is not an accurate representation of a chemical equation and thus it requires balancing.
Balanced Chemical Equation
A balanced chemical equation has an equal number of atoms of different elements in reactants and products.
No. of atoms of elements in Reactants = No. of atoms of elements in Products
Example1
Zinc reacts with Sulphuric Acid to form Zinc Sulphate + Hydrogen
Zn + H 2 SO 4 → ZnSO 4 + H 2
In this case,
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Zinc |
1 |
1 |
|
Hydrogen |
2 |
2 |
|
Sulphur |
1 |
1 |
|
Oxygen |
4 |
4 |
Since atoms of all elements are equal in Reactants and Products
It is a Balanced Chemical Equation
How to Balance an Unbalanced Chemical Equation?
Suppose we want to balance the following equation;
Example 1
H 2 + O 2 → H 2 O

Step 1
Let's note down current number of atoms of elements in reactants and products
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Hydrogen (H) |
2 |
2 |
|
Oxygen (O) |
2 |
1 |
Step 2
Choose element which is unbalanced
It is Oxygen in our case,
No. of atoms of Oxygen in Reactant is 2 and Product is 1
So in order to make them same, we multiply product H 2 O by 2
H 2 + O 2 → 2 H 2 O

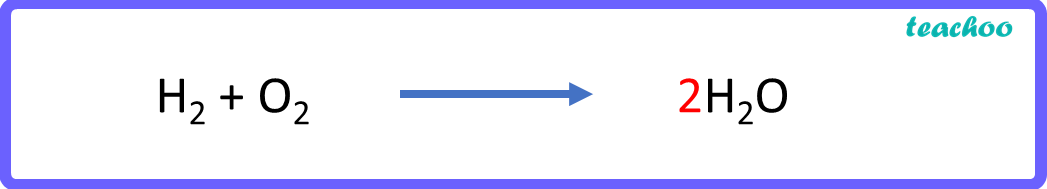
Now let us check the number of atoms in reactants & products again
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Hydrogen (H) |
2 |
4 |
|
Oxygen (O) |
2 |
2 |
Step 3
Now Number of Hydrogen atoms have become unequal
So we multiply H 2 by 2 to make it double
2 H 2 + O 2 → 2H 2 O

Now let us check the number of atoms in reactants & products once again
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Hydrogen (H) |
4 |
4 |
|
Oxygen (O) |
2 |
2 |
Since no. of atoms of reactants and products are equal
Equation is now balanced
Example 2
Balance the following chemical equation
Fe + H 2 O → Fe 3 O 4 + H 2

Step 1
Let's note down current number of elements in reactants and products
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Iron (Fe) |
1 |
3 |
|
Hydrogen (H) |
2 |
2 |
|
Oxygen (O) |
1 |
4 |
Step 2
We see that the number of Hydrogen atoms are balanced.
But Iron and Oxygen are not balanced
We start balancing from the beginning of the equation (from the reactants side)
There is 1 Fe atom on reactant side and 3 on product side
So we'll multiply Fe by 3 on the reactant side.
Now, the equation becomes as follows
3 Fe + H 2 O → Fe 3 O 4 + H 2

|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Iron (Fe) |
3 |
3 |
|
Hydrogen (H) |
2 |
2 |
|
Oxygen (O) |
1 |
4 |
Now, we move onto the next element, which is H
There are 2 H atoms on the reactant side and product side, hence it is balanced.
Next, we move onto O atoms.
There is 1 O atom on reactant side and 4 on product side
So we'll multiply H 2 O by 4 on the reactant side in order to balance O.
Now, the equation becomes as follows;
3 Fe + 4 H 2 O → Fe 3 O 4 + H 2

|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Iron (Fe) |
3 |
3 |
|
Hydrogen (H) |
8 |
2 |
|
Oxygen (O) |
4 |
4 |
Step 4
After balancing the last element on the reactant side (O in the above example)
We'll again go through the whole equation and check the balancing of every element from the beginning.
Checking 1st element - Fe
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Iron (Fe) |
3 |
3 |
Iron (Fe) is balanced
Checking 2nd element - H
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Hydrogen (H) |
8 |
2 |
Now, we've 8 atoms of H on the reactant side and 2 on the product side.
So, we'll multiply H 2 by 4 on the product side in order to balance H.
Now, the equation becomes as follows
3 Fe + 4 H 2 O → Fe 3 O 4 + 4 H 2

H is also balanced now.
Checking 3rd element - O
|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Oxygen (O) |
4 |
4 |
Oxygen (O) is balanced
Step 5
Now, we'll check the balancing of each atom once again from 1st to last element.
If all the elements are balanced, our balancing is done.
In this case,
Final balanced equation is;
3 Fe + 4 H 2 O → Fe 3 O 4 + 4 H 2

|
Element Name |
No of Atoms in Reactant |
No of Atoms in Product |
|
Iron (Fe) |
3 |
3 |
|
Hydrogen (H) |
8 |
8 |
|
Oxygen (O) |
4 |
4 |
Note: If the equation is still not balanced, repeat the steps till all elements are balanced.
Now, we know the exact number of atoms that are taking place in the reaction .
But, in order to make it more informative we can add the physical state of each component of the reaction.
As discussed earlier, this is done by writing the physical states in brackets next to the reactants and products.
We write the following symbols for corresponding states
|
State |
Symbol |
|
Solid |
s |
|
Liquid |
l |
|
Gas |
g |
|
Aqueous |
aq |
The above balanced equation can be re-written along with the physical states as follows;
3Fe (s) + 4H 2 O (g) → Fe 3 O 4 (s) + 4H 2 (g)
-+-4h2o-(g)-=-fe3o4-(s)-+-4h2-(g).png)
Note: The physical form (g) of H2O represents that H2o is taking part in the reaction as steam
Intext Question - Page 2 Q6
NCERT Exercise - Q4, Q5, Q6, Q7, Q8
