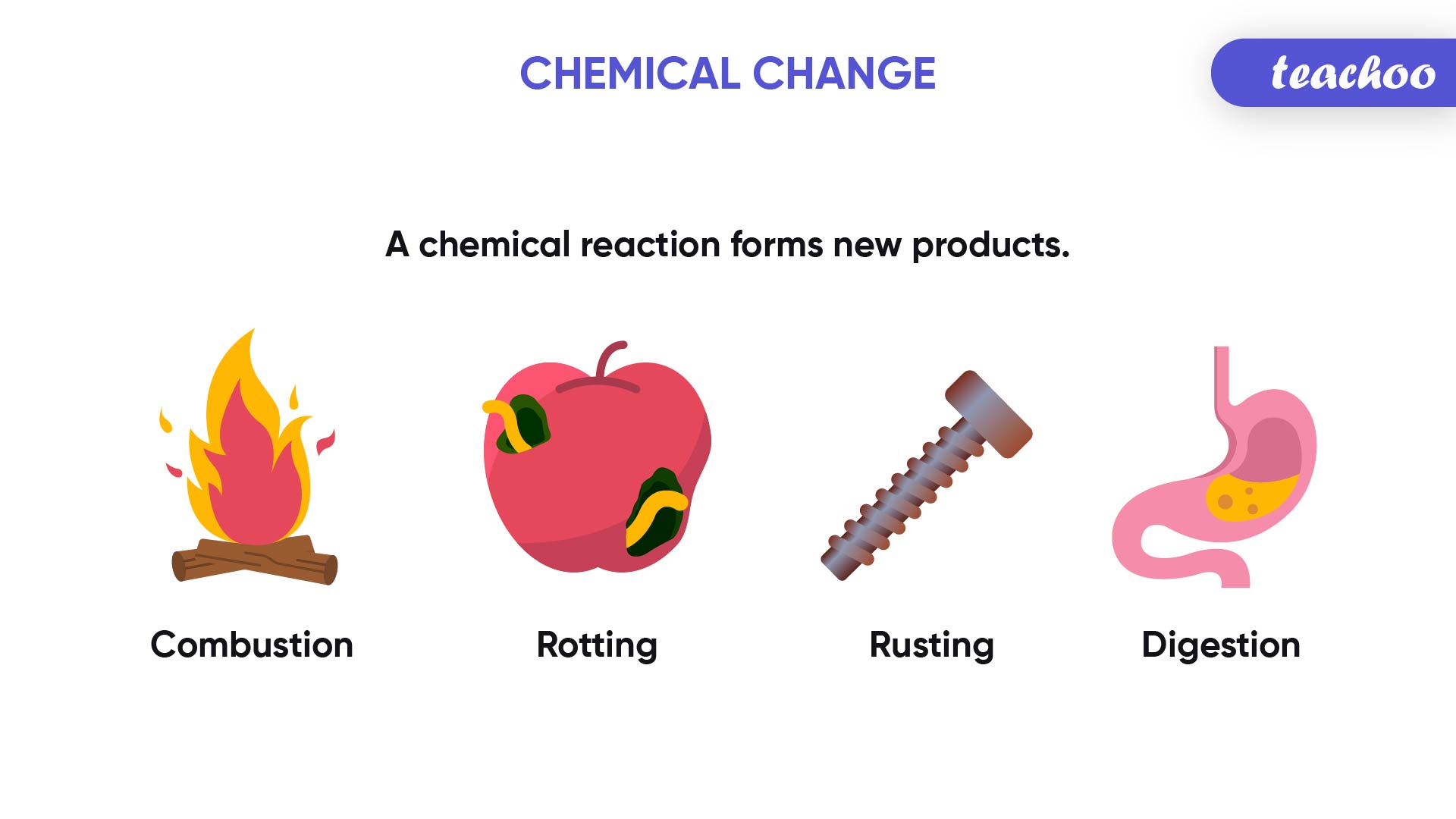
Take a look at the different processes taking place around you:
- Plants preparing food by the process of photosynthesis
- Iron structures turning reddish brown with time due to corrosion (rusting)
- Dirt in clothes getting removed on washing with soap and water
- Cake batter rising and getting cooked on baking
- Burning of wood at a campfire
All of these processes that we observe in our day to day lives are a result of Chemical Reactions.
What are Chemical Reactions?
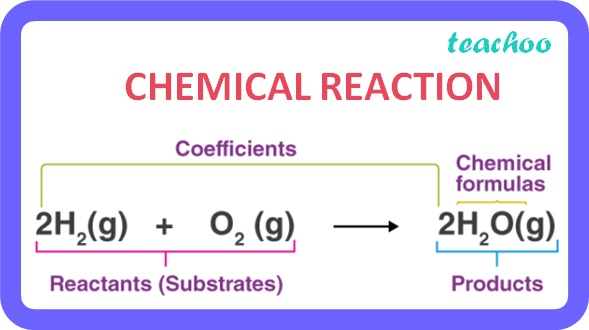
It is a process by which new substances with new properties are formed .
In a chemical reaction one or more substances combine to form new substances.
Example :
Hydrogen reacts with Oxygen to form Water
Hydrogen + Oxygen → Water
(Reactants) (Product)
Note
: When Hydrogen and Oxygen react, they form a new substance Water, hence it is a chemical reaction
Is chemical reaction a result of Physical change or chemical change?
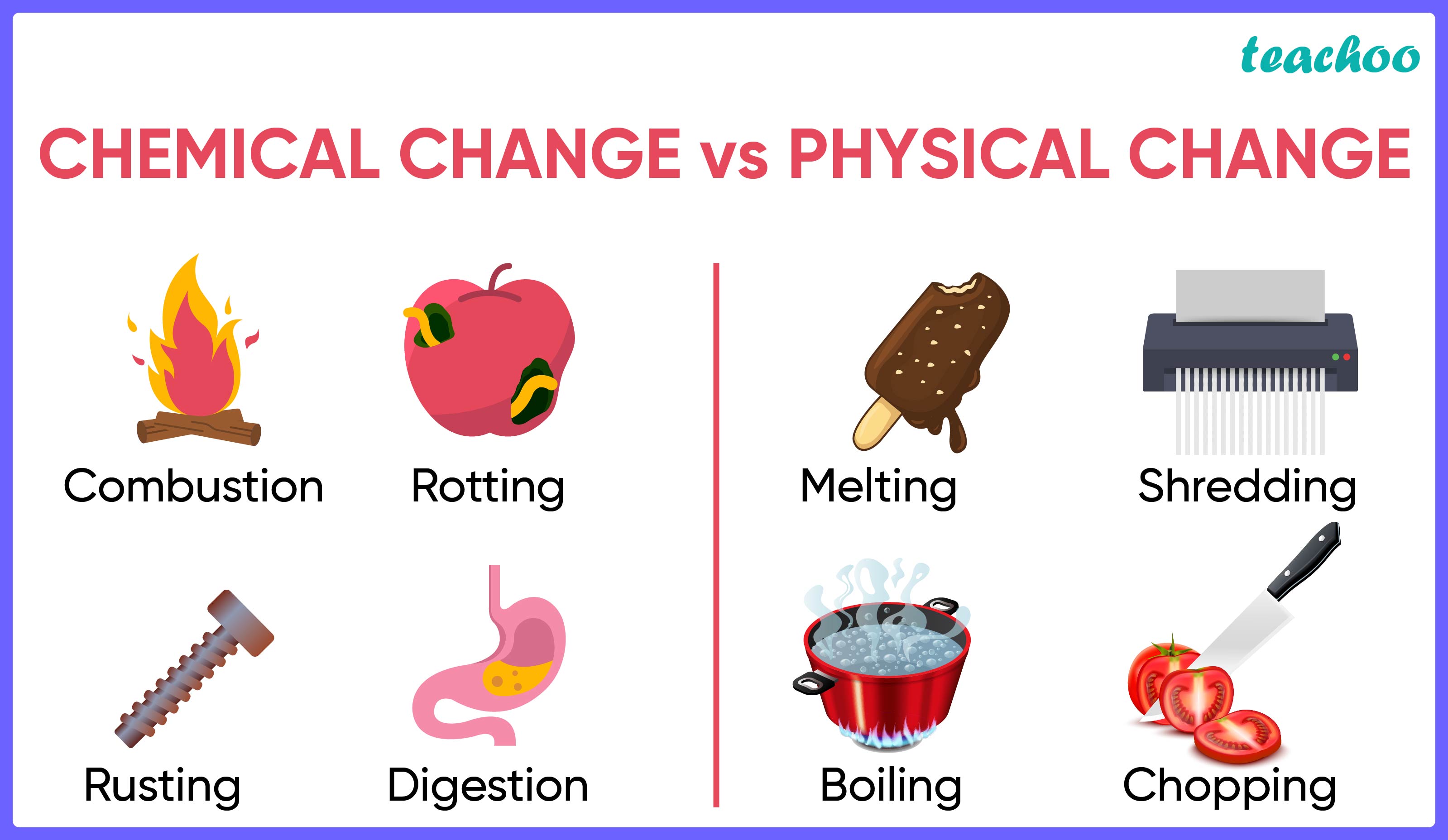
Chemical Reaction results in the formation of new substances.
Thus we can say, it occurs because of chemical changes
Explanation
|
PHYSICAL CHANGES |
CHEMICAL CHANGES |
|
No new substance is formed. |
New substances are formed. |
|
These changes are reversible |
These changes are normally irreversible |
|
Example: When ice melts into water, it is a physical change It is because ice and water are chemically same (both are H 2 O) We can convert ice to water and vice versa. Hence it is a physical change |
Example: Burning is chemical change When a paper is burnt, it turns into carbon This change cannot be reversed to get back paper Hence it is a chemical change |
Based on the above explanation of physical and chemical changes, we can confirm that chemical reactions occur as a result of chemical changes , as;
- New products are formed as a result of chemical reactions and
- The reaction is usually not reversible under the same conditions
Note : If the products can react to produce the reactants, then the reaction will be considered reversible.
Do elements change because of chemical reaction ?
The simple answer is, No
Atom of one element does not change into another element
They only rearrange to form new substances
Example
Magnesium + Oxygen → Magnesium Oxide
During this reaction, Magnesium Oxide is formed which contains only Magnesium and Oxygen element (and not any new element )
Note- Magnesium ribbon burns with a dazzling white flame and changes into white powder. The white powder formed is Magnesium Oxide.
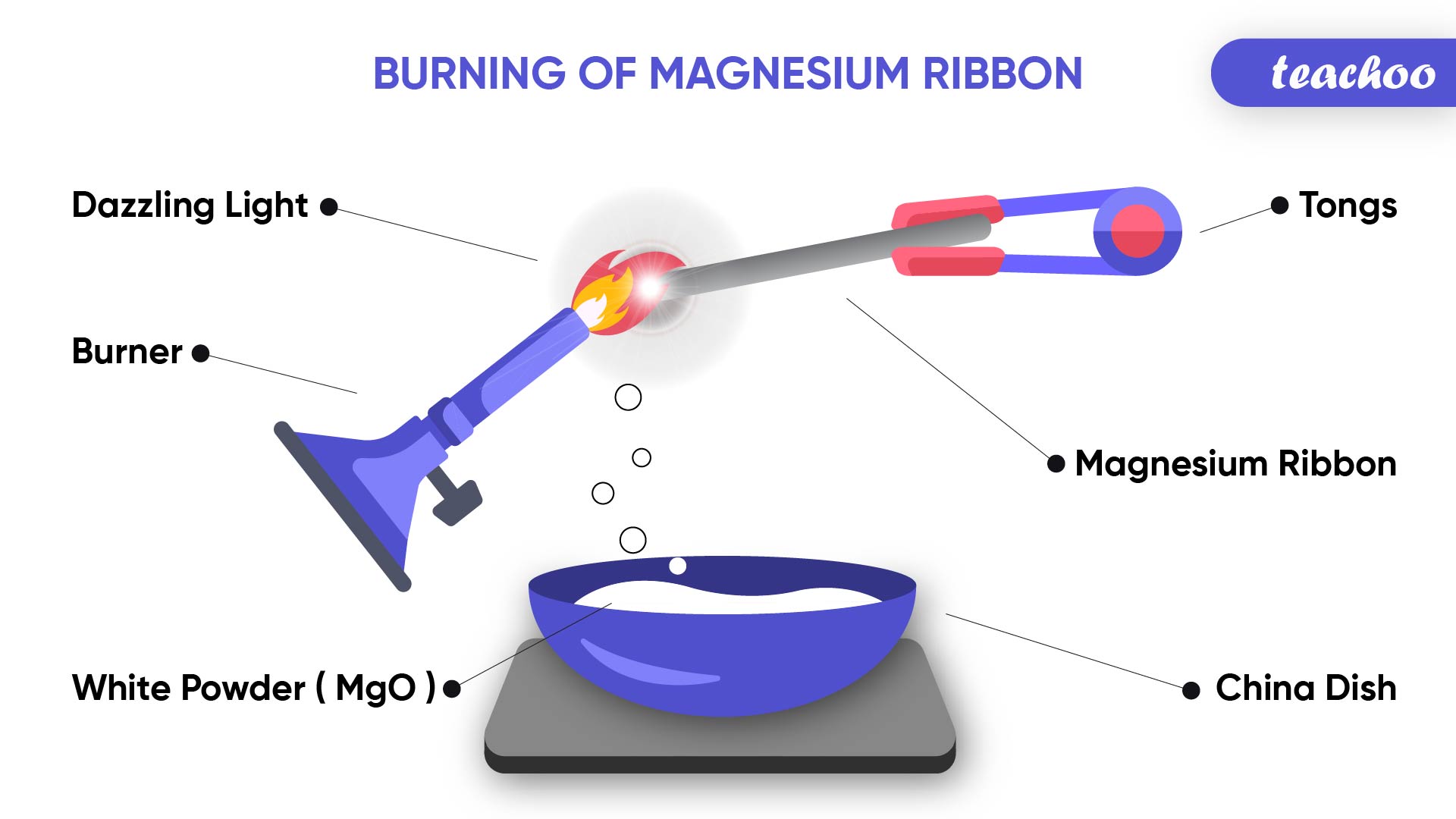
What are reactants and products?
The substances which undergo chemical change in a chemical reaction are called reactants .
The substances produced as a result of chemical reaction are called products
Example
Magnesium + Oxygen → Magnesium Oxide
In this case Magnesium and Oxygen are called Reactants
Magnesium Oxide is a Product
Magnesium + Oxygen → Magnesium Oxide
(Reactants) (Product)

How to find whether chemical change has taken place?
If a chemical change takes place, it usually leads to following:
- Change in state
- Change in color
- Evolution of Gas (Gas being formed)
- Change in Temperature
- Formation of precipitate
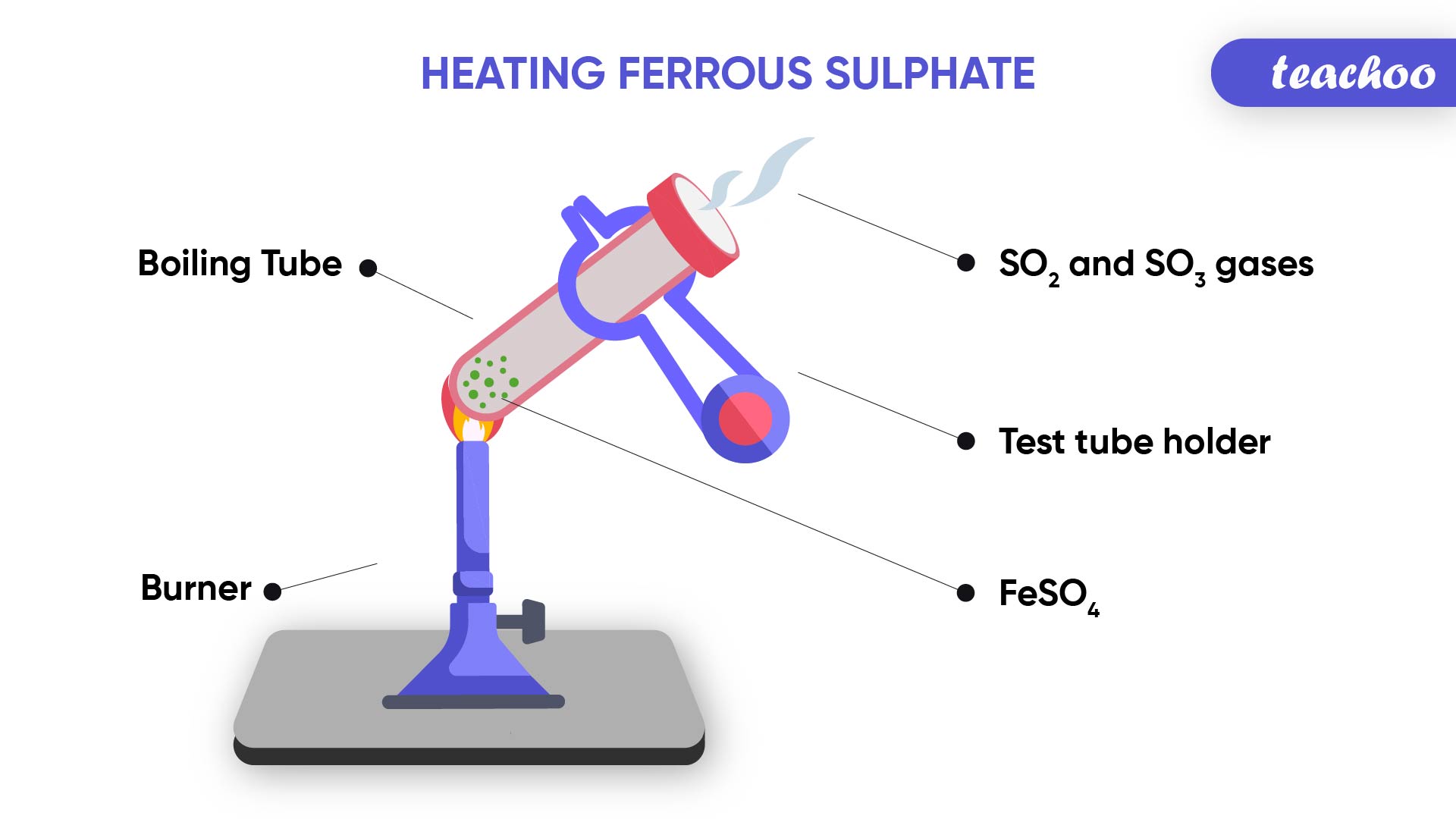
Example- Change in color of substances and evolution of gas in a chemical reaction
When we heat green coloured Ferrous Sulphate crystals in a boiling tube,
We observe that the green colour of Ferrous Sulphate changes .
We can also observe smell on burning of sulphur
This is because on heating,
Ferrous Sulphate breaks down into Ferric Oxide (solid) , Sulphur dioxide (gas) and Sulphur Trioxide(gas)
2FeSO 4 → Fe2O 3 + SO 2 + SO 3
(Reactant) (Products)

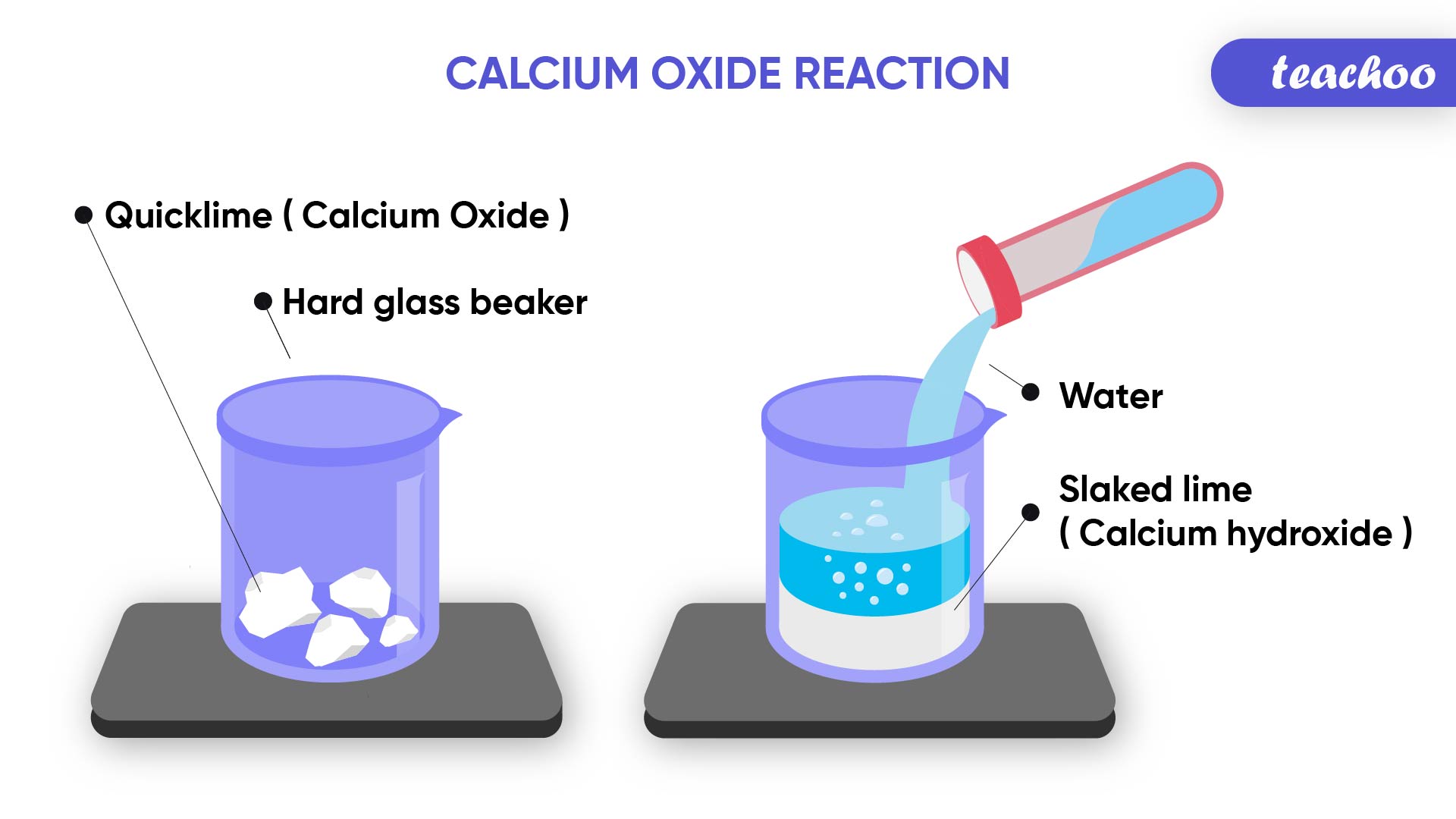
Example- Change in Temperature
Calcium Oxide (Quick Lime) reacts with Water to produce Calcium Hydroxide (Slaked Lime) and Heat
CaO + H 2 O → Ca(OH) 2
(Reactants) (Product)
Example- Formation of precipitate
When Sodium Sulphate solution is mixed with Barium Chloride solution
It forms Barium Sulphate precipitate and Sodium Chloride
(Insoluble solid is called precipitate)
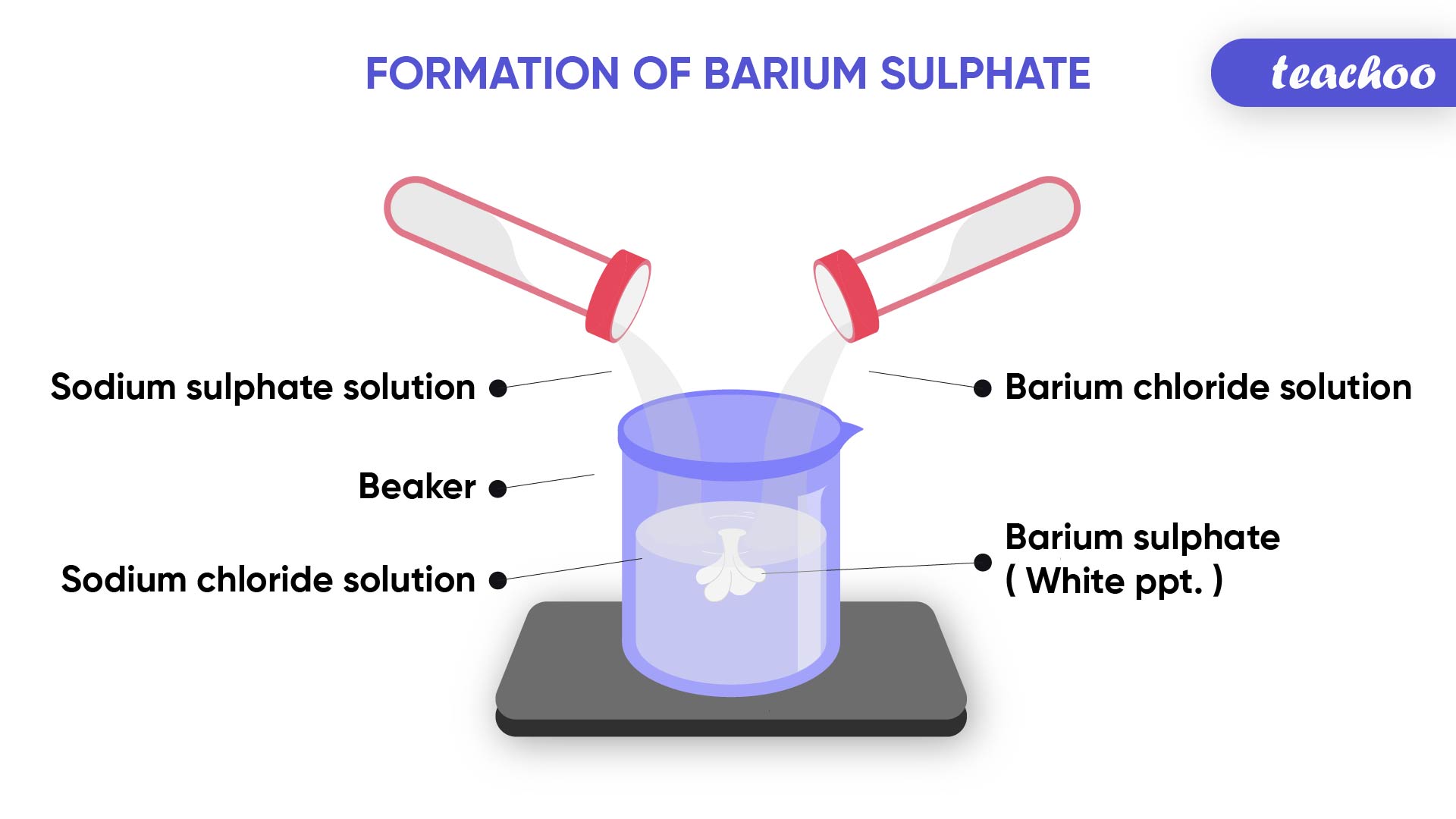
Note : This Barium Sulphate is formed because of the reaction of positively charged ions of Barium and negatively charged ions of sulphur
Intext Question - Page 6 Q1,
NCERT Exercise -
